Abstract
BACKGROUND: Despite the emerging data on the clinical effectiveness of the B cell receptor pathway inhibitors, complete remission is rarely induced and chronic lymphocytic leukemia (CLL) remains incurable. Reduced intensity/non-myeloablative conditioning (RIC/NMA) allogeneic hematopoietic cell transplantation (HCT) has been widely used in previously treated CLL patients and remains the only potentially curative therapy. The goal of this study is to develop a prognostic model and cytogenetic risk classification for CLL patients who undergo RIC/NMA HCT.
METHODS: We performed a retrospective data analysis of 606 patients with previously treated CLL reported to the Center for International Blood and Marrow Transplant Research between 2008 and 2014. To develop a prognostic model, the entire data set was randomly split into training (60%) and test sets (40%). Using the training set, a prognostic model was developed and validated in the test set. Primary endpoint was progression free survival (PFS). Cytogenetic risk was also classified using a multivariable model for PFS.
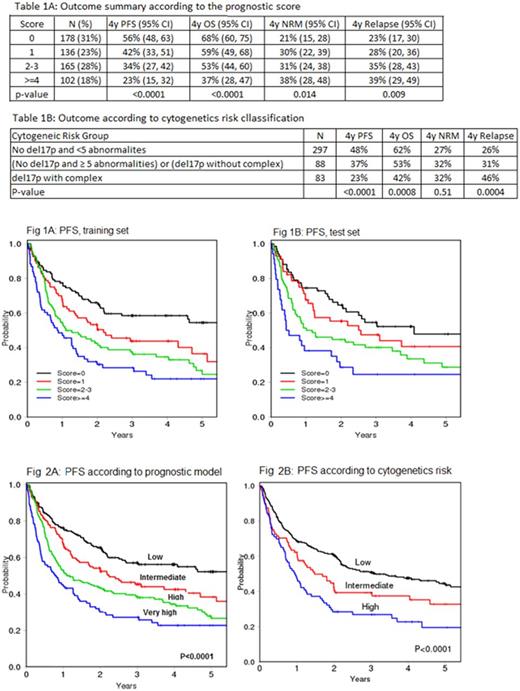
RESULTS: Of 606 patients who underwent RIC/NMA, the median age was58 (26, 73) and 72% were male. 32.2% had del17p and 27.3% had 3 or more cytogenetic abnormalities. The median follow up time among survivors was 47.3 months (range 3, 99). In multivariable Cox model for PFS, disease status at HCT (CR/PR vs no CR/PR), comorbidity score (0-1 vs ≥2), lymphocyte count at HCT (<2000 vs ≥2000/uL) and WBC at HCT (2-10 vs <2 or >10) were selected for the model. All other factors including age were not significant at the 0.05 level. Based on the hazard ratios (HRs) of these factors in the multivariable model, a score was assigned to each factor (1 if HR<1.5, 2 if HR>=1.5). Based on the summed score (range 0-6), patients were grouped into low (0), intermediate (1), high (2-3), and very high (≥4) risk categories. This result from the training set was validated in the test set (Fig.1A-B). For the entire cohort, the 4-year(y) PFS was 56% (95% confidence intervals [CI] 48-63), 42% (95% CI 33-51), 34% (95%CI 27-42), and 23% (95%CI 15-32) for low, intermediate, high and very high, respectively, p<0.0001 (Table 1A, Fig. 2A). This prognostic model also stratified OS, NRM and relapse.
In multivariable analysis, abnormal cytogenetics due to any of del13q, del11q, trisomy 12 (typically by FISH) or 3-4 abnormalities by karyotype had no impact on PFS. Only del17p and ≥5 abnormalities by karyotype showed inferior PFS. Based on HRs from the multivariable model, cytogenetic risk was classified as low (no del17p and <5 abnormalities), intermediate (no del17p with ≥5 complex or del17p without complex) and high (del17p with complex). The 4y PFS was 48% (95% CI 41-54), 37% (95% CI 27-48), and 23% (95% CI 14-33) for low, intermediate and high cytogenetics risk, respectively, p<0.0001 (Table 1B, Fig. 2B). When the prognostic score and cytogenetic risk were combined, patients with low prognostic score and low cytogenetic risk had prolonged PFS (4y PFS 59%, 95%CI 48-67) and OS (4y OS 72%, 95%CI 62-80) whereas patients with high-risk cytogenetics did poorly irrespective of the prognostic score (4y PFS 23%, 95%CI 14-33; 4y OS 43%, 95%CI 31-55).
CONCLUSIONS: In this large cohort of previously treated CLL patients who underwent RIC/NMA HCT in a multicenter setting, we successfully developed and validated a novel prognostic scoring system of HCT outcomes. This is the first score in the literature to risk stratify CLL patients at the time of HCT. We also developed a novel cytogenetic based risk stratification system. And finally we combined these two systems into one system. These data can be used for counseling patients, comparing data across different studies, and providing a platform for the evaluation of future interventions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal